On the surface, the difference between Truvada vs Descovy might look like splitting hairs. But this is not the case.
Many people assume that PrEP (pre-exposure prophylaxis) is the brand name of a medication for HIV prevention and treatment. However, PrEP is actually the name of the method for HIV prevention. The same goes for PEP (post-exposure prophylaxis), which is a treatment method for a person who is living with HIV.

There are currently two FDA approved PrEP medications available: Truvada and Descovy.
Both of these medications are prescribed for HIV treatment and prevention. But there are some key differences you should know if you are planning to consult your doctor about taking either one.
1. What Are These Medications?
Let’s start with the basics and give you some general information about Truvada and Descovy.
Truvada was the first PrEP medication approved by the FDA in 2012, but the medication was actually created and tested back in 2004.
It is a combination of two medications: emtricitabine and tenofovir. Truvada prevents HIV from spreading by blocking specific enzymes, which stops the virus from reproducing.
Descovy is a newer medication on the market as it was approved for PrEP in October of 2019.
This medicationuses emtricitabine, as well as tenofovir alafenamide.
Descovy is combined with antiretroviral agents to decrease the viral load of HIV, and it also blocks enzymes within the virus to prevent it from reproducing.
But while both of these medications serve the same purpose as a method of HIV prevention, there are some key differences to be aware of.
2. Both are Used for HIV Treatment and Prevention
Many people mistakenly assume that there is no difference in Descovy vs Truvada.
One of the key differences between these medications is the way the chemical makeup of each drug prevents HIV from spreading. Truvada is a TDF, which stands for tenofovir disoproxil fumarate. Descovy, on the other hand, is a TAF (tenofovir alafenamide). Both of these formulations are equally effective at preventing HIV transmission.
According to a medical study conducted by Kaiser Permanente, TAF medication and TDF were considered to be “non-inferior” to each other. However, this study did find that patients treated with TAF had a 53% numerical improvement in protection from HIV.
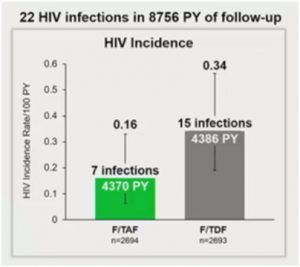
The only instance where TAF (Descovy) is considered to be more effective than TDF (Truvada) is when a boosting agent is administered as part of the therapy regiment.
A study published in the Journal of Virus Eradication stated that while there is no difference in the safety or effectiveness of these medications, TAF (Descovy) had a 2% higher rate of viral suppression when combined with a boosted antiviral regimen.
3. Recommended for Different Patients
PrEP medications are recommended for people at risk of contracting HIV through various forms of contact. For example, if a sexual partner is living with HIV or you have had sexual contact with partners who do not know their HIV status – you are at a higher risk of transmission.
Some professions are also at a higher risk of HIV transmission through contact with bodily fluids – such as people working in the medical field. Furthermore, anyone who has used shared injection needles should consider taking PrEP as a precaution to prevent HIV transmission.
However, the decision to take PrEP is ultimately between you and your doctor.
Currently, Truvada and Descovy are the only FDA approved medications for PrEP.But there are some significant differences in their formulation. Therefore, these medications are not interchangeable, nor are they prescribed to the same types of patients.
Neither medication is currently approved for children, but in some cases, it is prescribed to adolescents who meet certain criteria. This is mostly qualified by the patient’s weight. For PrEP treatment, patients must weigh at least 35 kg or 77 lbs.
If the medication is being used as a treatment for HIV-1 (PEP), then the weight requirements are lower – and it can be prescribed to pediatric patients.
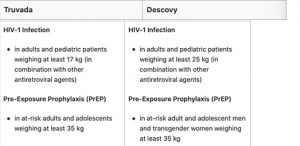
Truvada has been approved for adults and adolescents of all genders at-risk of HIV transmission.
Descovy, on the other hand, is only approved as PrEP medication in cisgender males and transgender women. This is because Descovy has not yet been evaluated for cisgender female or transgender male patients. However, further studies are being conducted to evaluate the drug’s efficacy and safety for female and transgender male patients.
Both of these medications do pose some common side effects, including:
- Nausea
- Weight gain or weight loss
- Diarrhea
These side effects typically occur during the “start-up” period and resolve after about three months of PrEP use. Truvada may cause cholesterol levels to decrease slightly, while Descovy could cause slight increases in LDL cholesterol and triglycerides.
Truvada can also cause significant and serious side effects in some patients. One medical report concluded that Truvada can lead to bone density loss. Therefore, it is not recommended for patients with osteoporosis or bone density issues.
It is important to note that a medical study concluded that any bone density loss caused by Truvada is reversible and the average decrease in bone mineral density is quite minimal.
Truvada can also affect the filtering capacity of the kidneys, which can lead to renal toxicity. Therefore, this medication is not advised for patients with pre-existing kidney conditions. Patients taking Truvada are required to have regular lab tests to monitor both kidney and liver functions.
Truvada may be prescribed at a lower dosage for patients exhibiting renal issues to decrease the risk of kidney disfunction.
Patients taking Descovy did not exhibit any of these side effects during clinical trials, and it currently does not have any testing requirements for patients. This generally makes Descovy a better choice for qualified patients who have pre-existing conditions that could be affected by Truvada. However, this is to be determined by a patient’s doctor on an individual basis.
Conclusion
HIV prevention and treatment has come a long way, and these medications have allowed people with HIV to live normal, healthy lives. They are also extremely effective as a PrEP method to protect people at a higher risk of HIV transmission.
Ultimately, Truvada and Descovy are both effectual medications and neither pose extreme risks. However, the PrEP medication that is right for you needs to be determined by your doctor.
If you would like to learn more about additional ways to protect yourself from HIV transmission or have additional questions about PrEP, connect with our team at PrEP Daily or check out other available resources.

